
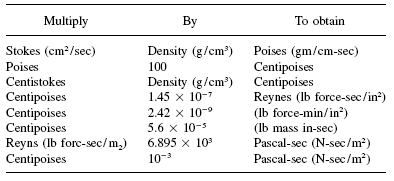
When dealing with viscosities, when we mention "viscosity," we actually mean dynamic viscosity.


In this article, we'll focus more on the viscosity of liquids, specifically on the kinematic viscosity and the dynamic viscosity of water. This weakening results in liquid molecules to move more freely and, therefore, with a lower viscosity. In liquids, when molecules start to move faster, their attraction from each other weakens. As a result, in gases, molecules experience more friction against each other, making them flow slower and become viscous. However, when we apply heat or additional thermal energy to our fluids, their molecules start moving faster. The attraction between the molecules of a viscous fluid is much higher than that of a less viscous fluid. We can also express viscosity as the internal friction of a fluid in motion. Maple syrup, a very viscous fluid, would pour slower than when you pour milk on your cereal as milk's viscosity is much lower. Imagine dripping maple syrup on your waffles for your breakfast. The higher the viscosity of a fluid (liquid or gas), the slower it traverses across a surface. Viscosity is the measure of a fluid's resistance to flow.


 0 kommentar(er)
0 kommentar(er)
